
Firefly and PC GAMESS-related discussion club

Learn how to ask questions correctly
Re^8: catalysis, negative energy activation in MP2 calculation
sanya
sanya@photonics.ru
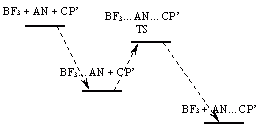
One more explanation was proposed by Dr. Granovsky. The explanation may be that the reactants that lead to the transition state you found are not acrylonitrile and 1-methylcyclopentadiene. In fact, your reaction proceeds in the liquid phase (acrylonitrile is liquid, BF3 is gas dissolved in the reactants, and 1-methylcyclopentadiene is probably liquid). The first step of your reaction is fast formation of AN...BF3 complex (barrierless process); its energy is lower than the energy of AN + BF3. Next, this complex reacts with 1-methylcyclopentadiene to form the transition state that gives the products. Therefore, the energy of your transition state should be calculated relative to the AN...BF3 complex + 1-methylcyclopentadiene rather than relative to the sum of individual components (AN, BF3, and 1-methylcyclopentadiene). See the attached picture.On Thu Feb 25 '10 6:01pm, Ronald Conol wrote
--------------------------------------------
>well. i never tried using HONDO in my study anyway. Ill input those keywords that you gave.. and ill update you if anything goes with it.
>thank you so much for your help.. i hope it will be over..
Probable reaction path
![[ Previous ]](/ceilidh-icons/ceil_prv.gif)
![[ Next ]](/ceilidh-icons/ceil_nxt.gif)
![[ Index ]](/ceilidh-icons/ceil_ind.gif) Thu Mar 4 '10 1:44am
Thu Mar 4 '10 1:44am
![[ Reply ]](/ceilidh-icons/ceil_rep.gif)
![[ Edit ]](/ceilidh-icons/ceil_edt.gif)
![[ Delete ]](/ceilidh-icons/ceil_del.gif) This message read 867 times
This message read 867 times


